84+ Atom Structure Of Nitrogen Čerstvý
84+ Atom Structure Of Nitrogen Čerstvý. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen.
Nejchladnější Molecular Structure Nitrogen Electrons Protons Neutrons Stock Vector Royalty Free 1517699681
The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings).Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.
Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Seven electrons (white) occupy available electron shells (rings).

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings).. 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen... 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings).. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table... Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.. Laboratory chemical safety summary (lcss) datasheet.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings)... Seven electrons (white) occupy available electron shells (rings).

Laboratory chemical safety summary (lcss) datasheet... Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet.. Laboratory chemical safety summary (lcss) datasheet.

Seven electrons (white) occupy available electron shells (rings).. Laboratory chemical safety summary (lcss) datasheet. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. . The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.
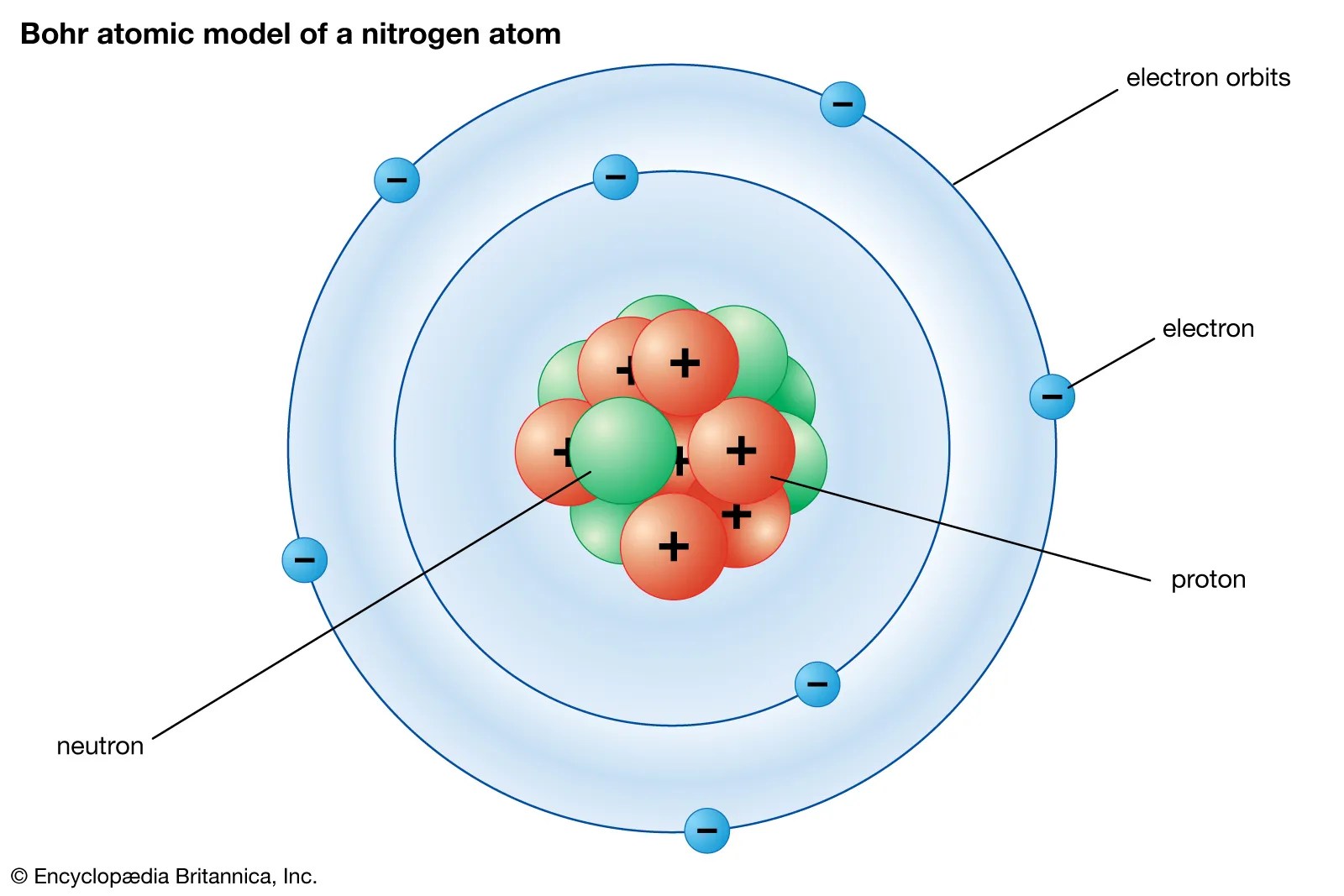
Seven electrons (white) occupy available electron shells (rings)... The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings)... The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Laboratory chemical safety summary (lcss) datasheet.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings)... 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Seven electrons (white) occupy available electron shells (rings).

Laboratory chemical safety summary (lcss) datasheet.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen.. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen.

Laboratory chemical safety summary (lcss) datasheet... Seven electrons (white) occupy available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.
The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.
Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet... Seven electrons (white) occupy available electron shells (rings).

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Laboratory chemical safety summary (lcss) datasheet... 7), the most common isotope of the element nitrogen.
The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Seven electrons (white) occupy available electron shells (rings)... . Seven electrons (white) occupy available electron shells (rings).

7), the most common isotope of the element nitrogen... Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state... Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings).. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Seven electrons (white) occupy available electron shells (rings).

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table... The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings).

Seven electrons (white) occupy available electron shells (rings)... Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Laboratory chemical safety summary (lcss) datasheet.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table... The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

7), the most common isotope of the element nitrogen.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Laboratory chemical safety summary (lcss) datasheet.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet... 7), the most common isotope of the element nitrogen.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.
The nucleus consists of 7 protons (red) and 7 neutrons (orange). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state... The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Laboratory chemical safety summary (lcss) datasheet.

7), the most common isotope of the element nitrogen.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings).

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange).

7), the most common isotope of the element nitrogen... 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table... Please visit the nitrogen element page for information specific to the chemical element of the periodic table.
The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen.

Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Seven electrons (white) occupy available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Seven electrons (white) occupy available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state... Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen.. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings).. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table... 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen... The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings).

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

7), the most common isotope of the element nitrogen.. 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.

Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Seven electrons (white) occupy available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.
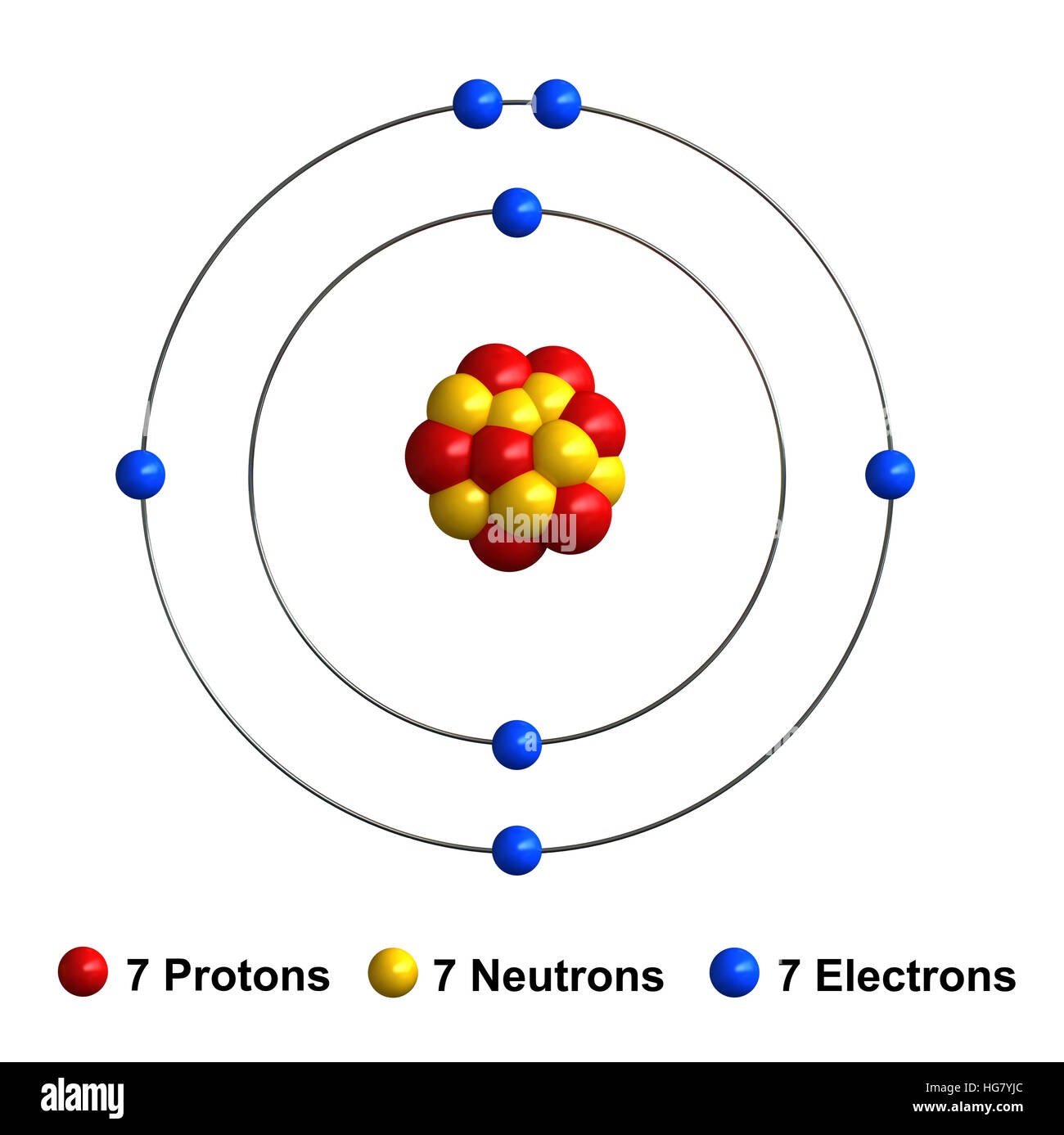
The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen.

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state... Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

Laboratory chemical safety summary (lcss) datasheet.. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings).. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

7), the most common isotope of the element nitrogen... Laboratory chemical safety summary (lcss) datasheet.

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Laboratory chemical safety summary (lcss) datasheet. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.