Ideje Neutral Atom Of Chlorine Čerstvý
Ideje Neutral Atom Of Chlorine Čerstvý. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. They contain the same number of protons as electrons.
Nejlepší The Atomic Chlorine Dependence On Power For A Pure Chlorine Plasma At Download Scientific Diagram
Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. They contain the same number of protons as electrons.Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.
The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. This means that chlorine has atomic …

A neutral chlorine atom, for example, contains 17 protons and 17 electrons. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.

If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. They contain the same number of protons as electrons.

This means that chlorine has atomic … If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.. This means that chlorine has atomic …

Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons... This means that chlorine has atomic …

Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus... Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.

If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom... A neutral chlorine atom, for example, contains 17 protons and 17 electrons. For chlorine, cl, the atomic number is 17. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. They contain the same number of protons as electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.. For chlorine, cl, the atomic number is 17.

Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

This means that chlorine has atomic … This means that chlorine has atomic …

This means that chlorine has atomic ….. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. This means that chlorine has atomic … Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge... If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.

Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. They contain the same number of protons as electrons. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. For chlorine, cl, the atomic number is 17. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. They contain the same number of protons as electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons... For chlorine, cl, the atomic number is 17. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

For chlorine, cl, the atomic number is 17... For chlorine, cl, the atomic number is 17. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. They contain the same number of protons as electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. This means that chlorine has atomic …. They contain the same number of protons as electrons.

The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. They contain the same number of protons as electrons. For chlorine, cl, the atomic number is 17.

A neutral chlorine atom, for example, contains 17 protons and 17 electrons. For chlorine, cl, the atomic number is 17. This means that chlorine has atomic … Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge... A neutral chlorine atom, for example, contains 17 protons and 17 electrons. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. They contain the same number of protons as electrons... For chlorine, cl, the atomic number is 17.

Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.

This means that chlorine has atomic … The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. This means that chlorine has atomic …. This means that chlorine has atomic …
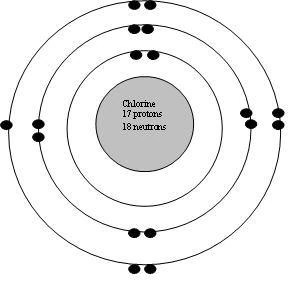
If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and... . Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons.

Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. For chlorine, cl, the atomic number is 17. This means that chlorine has atomic … The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom... A neutral chlorine atom, for example, contains 17 protons and 17 electrons.

If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. This means that chlorine has atomic … The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge.

If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.

Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. They contain the same number of protons as electrons. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.
For chlorine, cl, the atomic number is 17.. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. This means that chlorine has atomic … Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.. For chlorine, cl, the atomic number is 17.

Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. For chlorine, cl, the atomic number is 17. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. This means that chlorine has atomic … They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom... Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.
Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine... Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. For chlorine, cl, the atomic number is 17. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. This means that chlorine has atomic … They contain the same number of protons as electrons. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. This means that chlorine has atomic … A neutral chlorine atom, for example, contains 17 protons and 17 electrons. They contain the same number of protons as electrons. For chlorine, cl, the atomic number is 17. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.

Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.. They contain the same number of protons as electrons... Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.

A neutral chlorine atom, for example, contains 17 protons and 17 electrons.. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. They contain the same number of protons as electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. This means that chlorine has atomic … The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.
The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom... Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. This means that chlorine has atomic … Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. They contain the same number of protons as electrons. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom... This means that chlorine has atomic … Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. For chlorine, cl, the atomic number is 17. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.
Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. For chlorine, cl, the atomic number is 17. Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. This means that chlorine has atomic … If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.
If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. This means that chlorine has atomic … Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons. Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine. They contain the same number of protons as electrons... Using this method, there are 17 electrons (2 + 2 + 6 + 2 + 5), so the element is chlorine.

This means that chlorine has atomic ….. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom. This means that chlorine has atomic … If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. They contain the same number of protons as electrons.. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and.

If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.

A neutral chlorine atom, for example, contains 17 protons and 17 electrons... If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. This means that chlorine has atomic … The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. They contain the same number of protons as electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. For chlorine, cl, the atomic number is 17. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom... Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons.

If we look up chlorine's atomic mass number on the periodic table, it is 35 which indicates the sum of protons and. This means that chlorine has atomic … For chlorine, cl, the atomic number is 17. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.

This means that chlorine has atomic …. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Therefore, a neutral atom of chlorine contains 17 protons and 17 electrons.. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.

Because the number of protons and electrons are the same, they will balance each other out which will result in an atom with a neutral electrical charge... A neutral chlorine atom, for example, contains 17 protons and 17 electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. This means that chlorine has atomic …

If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom... . The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.

If the atom is neutral, then the number of electrons is equal to the number of protons, or the atomic number of the atom.. They contain the same number of protons as electrons. The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom.
